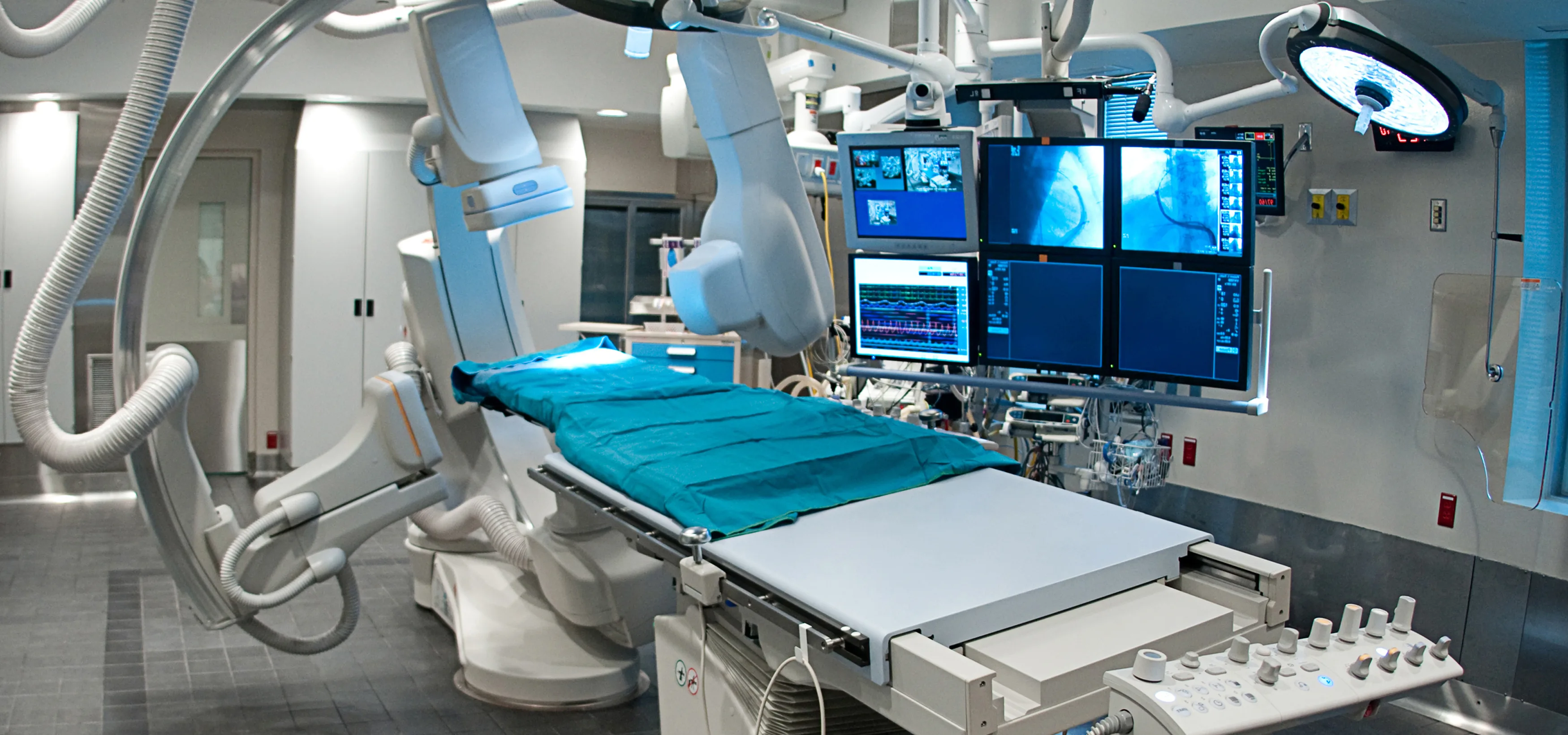
Medical Manufacturing — Trusted Partner in Vertically Integrated, Compliant Solutions
Precision medical manufacturing powered by vertical integration and global quality assurance.
Volex supports leading medical OEMs with vertically integrated manufacturing and complete system integration services, from printed circuit board assemblies (PCBAs) through full box builds, wiring harnesses and complete medical systems. Our manufacturing processes are structured to meet the highest global standards for safety, traceability and repeatability, ensuring that performance-critical medical devices are delivered reliably, on time and to precise specification.
As a trusted strategic partner to medical technology leaders, Volex combines deep technical expertise with a strong culture of compliance. Our global facilities operate under ISO 13485-certified quality systems and FDA 21 CFR 820 regulatory requirements, following GMP manufacturing practices with fully validated IQ/OQ/PQ processes to ensure consistent, controlled and repeatable production. Volex supports every stage of the medical device lifecycle with full lot traceability, robust documentation control and proven manufacturing discipline. Volex specialises in both high-volume and low-mix complex assemblies, ranging from diagnostic and imaging systems to patient care instrumentation, surgical robotics and laboratory equipment.
With a diversified global manufacturing footprint, Volex enables localised production strategies and helps mitigate tariff-related risks while maintaining business continuity. Our engineering and operations teams are experienced in navigating international medical device regulatory landscapes, managing sensitive supply chains, and supporting OEMs with qualification builds, design transfers and long-term lifecycle management.

Medical Applications
Dental devices
Hematology instruments
Imaging systems (X-ray, MRI, ultrasound, CT)
Life sciences instrumentation
Diagnostic systems and test equipment
Medical robotics and surgical systems
Single-use medical cables and consumables
Respiratory care devices
Therapeutic systems (oncology, cardiology, etc.)
Key Medical Benefits
Single-source partnership across subsystems and full systems
Assured compliance, traceability and validation across the product life cycle
Risk mitigation via global manufacturing footprint and localization
Accelerated time-to-market with design, prototyping, certification and volume production under one roof
Flexible scaling from small runs to high volume
Reduced complexity in supplier management and supply chain oversight
Medical Projects Engagement
Project Definition & Technical Alignment
Collaborate on design intent, patient safety standards and regulatory compliance, including ISO 13485, FDA 21 CFR 820, GMP expectations and MedAccred Accreditation requirements.
Design Collaboration & Prototype Development
Build early prototypes in cleanroom environments for functional and certification validation.
Pilot Production & Qualification
Controlled pilot runs under full traceability and sterilisation protocols.
Full Production Roll-Out
Vertically integrated manufacturing with global cleanroom assembly and validated supply chains.
Lifecycle Management & Continuous Optimisation
Ongoing support for product evolution, documentation updates and obsolescence control.
Medical Products & Services
Integrate mechanical, electrical, and electronic components into complex medical instruments.
Full medical system assemblies combining subsystems into ready-to-deploy units.
Control panels, actuation systems, instrumentation enclosures for medical devices.
Custom medical grade harnesses and cable assemblies (sterilizable, shielded, compliant).
Medical grade PCBAs for diagnostics, imaging, life science instruments.
Custom displays and HMIs for medical imaging, monitoring and device interfaces.
Control, computing, sensing modules for medical electronics and IoT health devices.
Base wire and cable products used in medical wiring and device build-outs (single and multi-conductor, insulation and jacket choices).
Contact Volex today to explore globally integrated manufacturing solutions tailored to the medical sector.